
CBERs Nicole Verdun tells a Friends of Cancer Research meeting on next-generation therapies that FDA has evolved in the last decade in its ability to ...

FDA commissioner Marty Makary says the agency is planning to release in the coming days a vaccine development framework to help with predictability fo...

FDA warns Malaysias BioAsia Worldwide about CGMP violations in its production of finished drugs, some of which may be regulated as cosmetics.

Cytokinetics reports topline results from its Phase 3 MAPLE-HCM clinical trial showing that aficamten significantly improved exercise capacity in pati...

FDA accepts for priority review a Regenxbio BLA seeking accelerated approval for gene therapy RGX-121 (clemidsogene lanparvovec) for treating Hunter s...

FDA warns Chinas Shantou S.E.Z. Baojie Industry about CGMP violations in its production of finished drugs, some of which may be regulated as cosmetics...
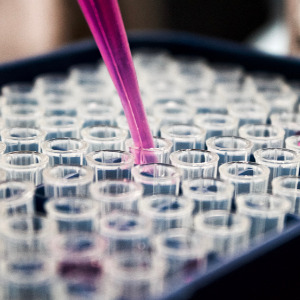
Biotech executives, investors, and consultants say some companies are moving early clinical trials overseas due to fears of instability and slowness a...

FDA approves a Merck NDA for Welireg (belzutifan), an oral drug for treating adults and pediatric patients aged 12 and older with locally advanced, un...