
Attorney Bradley Thompson says he is surprised and disappointed that FDA is not approving enough new consensus standards to support digital health and...

FDA announces a pilot program designed to expedite the review of new therapies addressing unmet medical needs.
FDA removes the clinical holds on three CARsgen Therapeutics clinical trials evaluating CT053, CT041, and CT071.

FDA agrees on a Phase 3 study design for Disc Medicines bitopertin and its use in treating a rare sunlight sensitivity disorder.
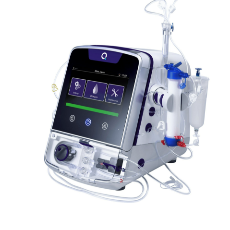
FDA clears a Quanta Dialysis Technologies 510(k) for the use of its Quanta Dialysis System in the home setting.
Mark Cubans Cost-Plus Manufacturing and Compounding facility in Dallas, TX, receives a Form FDA-483 based on findings from a recent inspection.
FDA accepts for review an Ionis Pharmaceuticals NDA for donidalorsen, an RNA-targeted treatment for preventing hereditary angioedema attacks.

FDA gives the green light to Phaxiam Therapeutics for a Phase 2 study (GLORIA) to evaluate its anti-Staphylococcus aureus phages in prosthetic joint i...