FDA clears a bioMrieux 510(k) for its Biofire Spotfire Respiratory/Sore Throat Panel Mini for detecting five of the most common viral and bacterial ca...

FDA says Aurobindo has recalled one lot of its OTC Healthy Living migraine medication that was distributed through Amazon without the FDA-approved lab...
FDA publishes a Veterinary International Conference on Harmonization revised guidance on residual solvents used in animal drugs.

Federal Register notice: FDA makes available a final guidance entitled Real-World Data: Assessing Electronic Health Records and Medical Claims Data To...
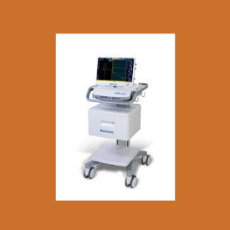
Medtronics Xomed unit recalls (Class 1) its NIM Vital Nerve Monitoring system due to the potential for false negative responses.
FDA publishes an immediately effective guidance with its recommendations for investigational and licensed Covid-19 convalescent plasma.

Two stakeholders call for changes to an FDA draft guidance on safety testing of human allogeneic cells expanded for use in cell-based medical products...

House Republican leaders postpone a floor vote this week on FDAs fiscal year 2025 budget.