
Federal Register notice: FDA makes available a draft guidance entitled E2D(R1) Post-Approval Safety Data: Definitions and Standards for Management and...
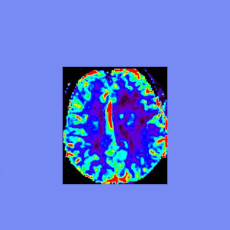
A Science investigative report looks at issues found by FDA in a laboratory analysis of results from trials of Cassavas potential Alzheimers drug simu...

Republican Sen. Bill Cassidy asks interested parties to submit their ideas for congressional action to reform the regulation of clinical tests.

FDA approves a Madrigal Pharmaceuticals NDA for Rezdiffra (resmetirom) for treating adults with noncirrhotic non-alcoholic steatohepatitis.

FDA publishes a draft ICH guidance on post-marketing individual case study reporting.

FDA medical reviewers ask the Oncology Drug Advisory Committee to weigh the benefits of the benefits and serious risks of Gerons imetelstat to treat s...

FDA asks the Oncologic Drugs Advisory Committee to evaluate the higher risk of early deaths in trials supporting sBLAs for Celgenes Abecma and Janssen...

Mintz attorney Benjamin Zegarelli says FDA needs to develop creative ways to regulate software as a medical device while it waits for Congress to give...