
U.S. Senator Tammy Baldwin (D-WI) writes the four biggest drug companies marketing asthma inhalers urging them to stop unfairly blocking generics from...

Merck says its Phase 3 KEYNOTE-564 trial evaluating Keytruda (pembrolizumab) as an adjuvant treatment in patients with renal cell carcinoma saw an imp...

Philips Respironics and FDA reach an agreement on the terms of a consent decree over the companys struggles to resolve issues surrounding its troubled...
FDA issues a guidance entitled Human Gene Therapy Products Incorporating Human Genome Editing.

Azurity Pharmaceuticals recalls one lot of narcolepsy drug Zenzedi CII (dextroamphetamine sulfate tablets, USP) 30 mg after a report was received that...

Merck says data from its Phase 3 AMBASSADOR (A031501)/KEYNOTE-123 trial evaluating Keytruda (pembrolizumab) are favorable for the therapy as a potenti...
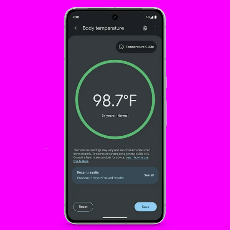
FDA grants Google a de novo marketing authorization for its new body temperature app on its latest Pixel 8 Pro smart phone.

FDA releases a document entitled Best Practices for FDA Staff in the Postmarketing Safety Surveillance of Human Drug and Biological Products.