
CBER says Lewisburg, TN-based Vitacell Biologics is illegally marketing cellular and exosome products.
FDA releases the form FDA-483 with two inspection observations issued to Columbus, OH-based STAQ Pharma of Ohio.
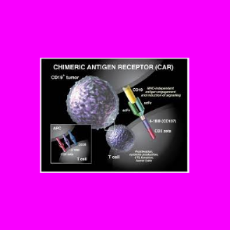
CBER executives say FDA is aware of a relatively small number of cases in which people treated with CAR-T products developed secondary cancers that ma...

FDA tells Liquidia Corp. that its earlier user fee review action target date of 1/24 will be missed on the companys NDA for Yutrepia (treprostinil) in...

A Sheppard Mullin report describes several FDA issues that life sciences companies can expect to face in 2024.

FDA issues a finalized guidance on updating ANDA labels following revisions to the reference-listed drug label.

CDER and CBER say they have accepted the first artificial intelligence, digital health technology, and neuroscience project in the ISTAND pilot for de...

FDA publishes a comprehensive Veterinary International Conference on Harmonization guidance on good manufacturing practice for animal drug APIs.