
FDA approves a Sandoz BLA for Tyruko (natalizumab-sztn), the first biosimilar to Biogens Tysabri (natalizumab) injection for treating adults with rela...

FDA says its Endocrinologic and Metabolic Drugs Advisory Committee will meet 9/21 to review and vote on an Intarcia Therapeutics NDA for ITCA 650 (exe...
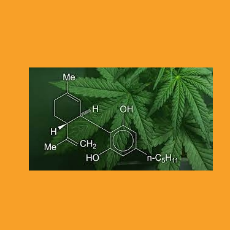
The Council for Federal Cannabis Regulation says FDA should use its enforcement discretion to regulate cannabis products now while working with Congre...

FDA issues a warning against using Dr. Bernes MSM Drops 5% Solution and LightEyez MSM Eye Drops Eye Repair due to bacterial or fungal contamination....

Federal Register notice: FDA extends the comment period for a 7/14 notice that announced the availability of a draft guidance entitled Manufacturing C...

Federal Register notice: FDA announces an 11/29-30 public workshop entitled Workshop to Enhance Clinical Study Diversity.

Draeger Medical recalls its Carina Sub-Acute Care Ventilators due to the presence of contaminants in the devices airpath.

Rep. Debbie Dingell asks FDA and CDC to answer questions about recent cases of tuberculosis linked to contaminated Aziyo Biologics bone grafts.