
FibroGen discontinues its ZEPHYRUS-2 Phase 3 trial of pamrevlumab in patients with idiopathic pulmonary fibrosis after topline data from the earlier Z...

Federal Register notice: FDA posts a draft guidance entitled Psychedelic Drugs: Considerations for Clinical Investigations.

ReShape Lifesciences files a PMA supplement for the companys next generation Lap-Band 2.0 for long-term treatment of obesity.
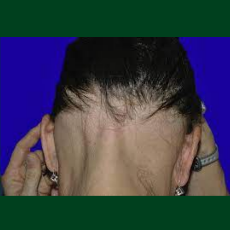
FDA approves a Pfizer NDA for Litfulo (ritlecitinib), a once-daily oral treatment for individuals 12 years of age and older with severe alopecia areat...

FDA extends its review of UCB's psoriasis drug bimekizumab for reasons the company did not disclose.

FDA clears a CS Medical 510(k) for its Ethos Automated Ultrasound Probe Cleaner Disinfector.

FDA lifts a Pharvaris clinical hold on a deucrictibant clinical trial for the on-demand treatment of hereditary angioedema attacks.

Citing potential liver injury concerns, Pfizer says it is terminating the development program for glucagon-like peptide-1 receptor agonist anti-diabet...