
FDA issues Incyte a complete response letter for Jakafi (ruxolitinib) extended-release tablets, a JAK1/JAK2 inhibitor for once-daily use in treating c...

Federal Register notice: FDA establishes a docket to solicit comments on factors that should be considered when reviewing risk evaluation and mitigati...

An FDA advisory committee votes 9 to 0 that Biogens amyotrophic lateral sclerosis drug tofersen should be considered for accelerated approval.

As required by the PDUFA 7 agreement, FDA publishes a framework for how it will implement a multifaceted program for using digital health technologies...
FDA adds the Xtrava Health SPERA Covid-19 test to the list of tests expected to have a reduced performance with certain Covid-19 Omicron sub-variants.
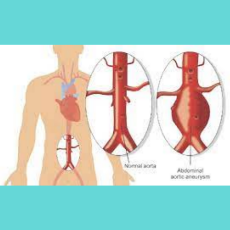
FDA-funded research finds that unibody endografts are not non-inferior to non-unibody devices, leading to updated FDA labeling.

FDA reminds patients and healthcare providers about risks associated with recalled Exactech replacement joint devices that were packaged in defective ...

Regeneron and Sanofi say an investigational Phase 3 trial of Dupixent in some COPD patients met all key primary and secondary endpoints.