
Pharmaceutical Research and Manufacturers of America tells the White House Office of Science and Technology Policy its three suggestions for enhancing...
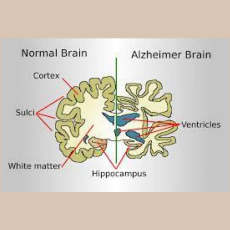
Roche and Eli Lilly collaborate to develop Roches Elecsys Amyloid Plasma Panel, a blood test designed for making earlier diagnoses of Alzheimers disea...

The CBER FY 2022 biological product deviation reporting annual summary says there was a 3% increase in reports submitted over FY 2021.

A new University of Colorado Boulder study points to the need for FDA to work harder to follow and consider further regulating social media micro-infl...

FDA approves an expanded indication for Regeneron Pharmaceuticals Evkeeza (evinacumab-dgnb) as an adjunct to other lipid-lowering therapies to treat c...

Federal Register notice: FDA determines for patent extension purposes the regulatory review period for Novo Nordisks Esperoct (antihemophilic factor (...

Federal Register notice: FDA amends certain medical device regulations to update mailing addresses and docket numbers.

FDA approves a Sandoz supplemental BLA for citrate-free Hyrimoz (adalimumab-adaz) injection, a biosimilar version of Abbvies Humira.