
FDA denies a 3/2021 petition filed by Loeb & Loeb that asked the agency to take specific actions concerning the adequacy of safety labeling for Incyte...

Democrat members of the House Energy and Commerce Committee vote Rep. Anna G. Eshoo (D-CA) as ranking Democrat on the Health Subcommittee.

LivaNova (TandemLife) recalls its LifeSPARC Controller due to a problematic software update that was designed to address a previous software malfuncti...

Federal Register notice: FDAs Office of Operations (OO), Office of Enterprise Management Services (OEMS) modifies its organizational structure to add ...

FDA grants Eli Lilly accelerated approval for Jaypirca (pirtobrutinib) for treating certain relapsed or refractory mantle cell lymphoma patients.

House leaders tap Representative Brett Guthrie (R-KY) to serve as chair of the House Energy and Commerce Committees Health Subcommittee, which oversee...

Two Hyman, Phelps & McNamara attorneys describe requirements in the new Food and Drug Omnibus Reform Act to improve clinical trial diversity.
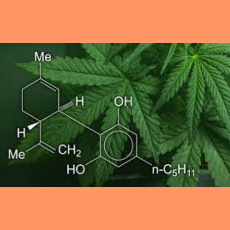
FDA principal deputy commissioner Janet Woodcock says the agency will work with Congress to develop a new cross-agency regulatory framework for cannab...