
FDA posts a seven-item Form FDA-483 from an inspection of New England Life Care (dba Advanced Compounding Solutions) earlier this year.

An FDA advisory committee unanimously votes to not recommend approval of a Y-mAbs Therapeutics BLA for I-omburtamab.
FDA clears a Fresenius Medical 510(k) to implement changes to its 2008T hemodialysis machines that addresses an earlier FDA safety alert.

An FDA advisory committee votes to recommend approval of GlaxoSmithKlines anemia drug daprodustat for use in treating chronic kidney disease in adult ...
Attorney Michelle Yeary says a California state court granted Medtronic summary judgment on several product liability claims involving a spinal cord s...

FDA announces the retirement of CDER Office of Compliance director Don Ashley.
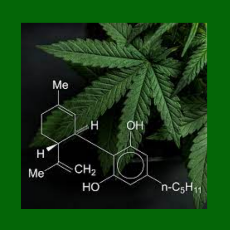
FDA principal deputy commissioner Janet Woodcock says the agency is exploring what flexibility it has in reviewing the science and health benefits of ...

Federal Register notice: FDA announces an Emergency Use Authorization for Abbott Molecular and its Alinity m MPXV monkeypox diagnostic test.