
FDA approves a Revance Therapeutics BLA for injectable Daxxify (daxibotulinumtoxinA-lanm) for temporarily improving moderate-to-severe frown lines.

FDA issues a draft guidance intended to improve the consistency of labeling for nonprescription drugs.
In an unusual second advisory committee meeting in six months, panel members reverse their March vote and recommend approval for Amylyxs amyotrophic l...

FDA releases a Form FDA-483 with seven observations from an inspection at Koreas Hugel drug substance and drug product manufacturing facility.

FDA publishes a draft guidance on general clinical pharmacology considerations for pediatric studies of drugs, including biologics.
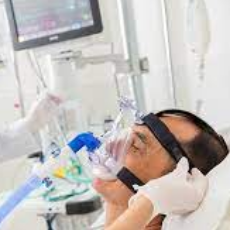
Philips Respironics recalls 17 million masks used with bilevel positive airway pressure machines and continuous positive airway pressure machines due ...

Federal Register notice: FDA announces a 10/6 advisory committee meeting to discuss a Veru Inc. request for an emergency use authorization for tubulin...

Federal Register notice: FDA announces that a product was approved using a priority review voucher Genentechs Vabysmo (faricimab-svoa).