
FDA denies most requests in a Salix Pharmaceuticals petition asking for restrictions on approval of ANDAs citing its Xifaxin as the reference-listed d...

Bayer agrees to pay $40 million to the federal government and 20 states to resolve two whistleblower False Claims Act suits over three of its drugs.

FDA releases its 2022 report on advancing regulatory science with updates to the focus areas identified in the 2021 regulatory science report.
FDA accepts for review a Bausch & Lomb and Novaliq NDA for NOV03 (perfluorohexyloctane), an eye drop therapy for treating the signs and symptoms of dr...
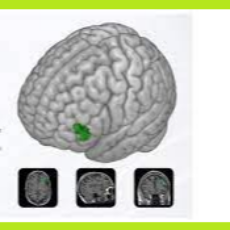
FDA clears a Magnus Medical 510(k) for the Saint Neuromodulation System for treating major depressive disorder in adults who have failed to achieve sa...

FDA approves Fresenius Kabis Stimufend biosimilar for Amgens Neulasta.

FDA reviewers dismiss new analyses and data from Amylyx that were intended to provide confirmatory evidence to support approval of its amyotrophic lat...

Federal Register notice: FDA announces the issuance of a priority review voucher to BioMarin Pharmaceutical for a rare pediatric disease product appli...