
Federal Register notice: FDA makes available a draft guidance entitled M12 Drug Interaction Studies.

Federal Register notice: FDA sends to OMB an information collection revision entitled Review Transparency and Communication for New Molecular Entity N...

Federal Register notice: FDA announces the issuance of one, and revocation of four Emergency Use Authorizations issued to STS Lab Holdco (a subsidiary...

Moderna files a patent infringement lawsuit against Pfizer/BioNTech in Massachusetts federal court over their Comirnaty Covid-19 vaccine.
FDA makes available two International Council for Harmonization draft guidances on drug analytical procedures.

FDA removes N95 respirators from its medical device shortage list, which it says signals that demand or projected demand for this type of face protect...
FDA releases a final guidance entitled E14 and S7B Clinical and Nonclinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential Qu...
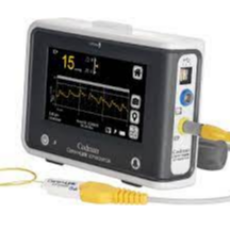
Integra LifeSciences begins a global product removal of all CereLink intracranial pressure monitors after customer reports were received about devices...